The role of genetics in dental caries is garnering increased attention in scientific circles. Research indicates that genetic factors greatly influence enamel strength, saliva production, and oral microbiome composition, all of which are essential in cavity development. Specific genetic markers have been identified that correlate with increased susceptibility to cavities. This raises intriguing questions about the potential for personalized dental care strategies that incorporate genetic information. Can genetics overshadow lifestyle factors in cavity prevention?
Understanding Dental Caries: A Brief Overview
Dental caries, commonly referred to as cavities, represent a complex interplay between microbial activity, dietary sugars, and host factors leading to tooth demineralization. This multifactorial process involves the acidogenic bacteria, primarily Streptococcus mutans, metabolizing fermentable carbohydrates, particularly sucrose, to produce acids that dissolve the tooth’s mineral content. Effective cavity prevention necessitates a thorough approach incorporating rigorous dental hygiene practices. These include mechanical plaque control through regular brushing with fluoride toothpaste, interdental cleaning, and routine dental check-ups. Additionally, reducing the intake of dietary sugars limits substrate availability for cariogenic bacteria, thereby mitigating acid production. Empirical evidence supports the integration of antimicrobial agents and fluoride treatments to enhance remineralization and inhibit microbial colonization, reinforcing the foundational role of preventive strategies in dental caries management.
The Role of Genetics in Oral Health
Emerging research underscores the significant influence of genetics on oral health, shaping susceptibility to dental caries and other oral conditions. Genetic predisposition is a pivotal factor, influencing individual variations in oral microbiome composition and immune response. Studies demonstrate that specific genetic markers can affect saliva composition, impacting the oral microbiome’s balance and pathogen resistance. Additionally, genetic variations may dictate host response to bacterial colonization, exacerbating susceptibility to cariogenic bacteria. Research utilizing genome-wide association studies has identified genes linked with caries risk, elucidating pathways related to sugar metabolism and immune response. Understanding these genetic influences enhances predictive models for oral health risks, potentially guiding personalized prevention strategies. This genetic framework underscores the complex interplay between inherited traits and environmental factors in oral health outcomes.
Tooth Enamel: Nature’s Protective Shield
Tooth enamel, the hardest substance in the human body, is primarily composed of hydroxyapatite crystals, whose structural integrity is critical for protective function against cariogenic challenges. Genetic variations can influence the mineralization process and subsequently alter enamel’s resistance to acid erosion and microbial attack. Understanding these genetic factors provides insight into the variability in enamel robustness among individuals and underscores its essential role in dental health maintenance.
Enamel Composition Factors
While often overlooked in discussions about dental health, the composition of tooth enamel plays a critical role in its ability to protect against cavities and decay. Enamel strength is mainly determined by its mineral content, chiefly hydroxylapatite, which contributes to its resilience. Variations in enamel composition can influence susceptibility to caries, as stronger enamel offers enhanced resistance to acid erosion. Genetic predisposition can affect the mineralization process, thereby impacting enamel formation and integrity. Studies indicate that genes associated with enamel development, such as AMELX, ENAM, and MMP20, contribute to these compositional differences. Consequently, individuals with certain genetic backgrounds may inherently possess weaker enamel, predisposing them to higher cavity risk. Understanding these factors is essential for developing personalized preventive strategies in dental care.
Genetic Enamel Variations
A significant body of research underscores the role of genetic variations in the composition and strength of tooth enamel, highlighting its function as a critical protective barrier against dental caries. Variations in specific genes, such as AMELX, ENAM, and MMP20, have been linked to alterations in enamel thickness and structure. Genetic mutations in these genes can lead to hypoplasia or hypomineralization, conditions that compromise enamel integrity. Studies employing genome-wide association analyses have identified single nucleotide polymorphisms (SNPs) that correlate with enamel defects, suggesting a hereditary component influencing enamel resilience. Additionally, research indicates that these genetic mutations may affect the amelogenesis process, ultimately impacting the enamel’s ability to withstand cariogenic challenges. Understanding these genetic factors is essential for developing targeted preventative strategies.
Protective Enamel Function
Enamel serves as the primary defensive barrier of teeth, offering essential protection against mechanical and chemical challenges. Its robustness is largely attributed to enamel strength, a function of its highly mineralized matrix mainly composed of hydroxyapatite crystals. This matrix guarantees resilience against mastication forces and acidic environments induced by dietary habits or microbial activity. Enamel thickness, a vital determinant of its protective efficacy, varies among individuals and can be influenced by genetic factors. Thicker enamel typically enhances resistance to wear and decay, providing a more formidable shield against cariogenic conditions. Conversely, reduced enamel thickness may predispose teeth to increased vulnerability. Investigating the genetic underpinnings influencing enamel’s structural properties remains pivotal in understanding individual susceptibility to dental caries.
Saliva Composition and Its Impact on Cavity Formation
Saliva composition, influenced by genetic factors, plays a critical role in the prevention and formation of dental cavities. Research indicates that variations in the concentrations of calcium, phosphate, and bicarbonate in saliva contribute to its protective properties against enamel demineralization. Consequently, understanding these genetic determinants and their impact on saliva’s biochemical makeup is essential for evaluating individual susceptibility to cavity development.
Genetic Influence on Saliva
While often overlooked, the genetic composition of saliva plays a vital role in oral health, particularly in the formation of cavities. Saliva production is influenced by numerous genetic factors, affecting both its quantity and biochemical components. Variations in genes associated with salivary gland function can lead to altered saliva production, impacting its ability to neutralize acids and wash away food particles. Genetic mutations may further modify the concentration of key proteins and enzymes within saliva, which are essential for maintaining oral pH balance and inhibiting bacterial growth. Studies have identified specific genetic markers linked to altered salivary amylase levels, which can influence starch breakdown and subsequent sugar availability for oral bacteria. Consequently, genetic predispositions greatly contribute to individual differences in cavity susceptibility.
Saliva’s Protective Properties
A shield of defense within the oral cavity, saliva is a complex fluid that plays an essential role in mitigating the risk of cavity formation. Saliva functions as a primary protective barrier, employing its unique composition to neutralize acids produced by oral bacteria. The presence of bicarbonate ions in saliva contributes to maintaining a neutral pH, thereby limiting enamel demineralization. Saliva enzymes, especially amylase and lipase, facilitate the initial breakdown of carbohydrates and fats, reducing substrate availability for bacterial metabolism. Additionally, antimicrobial proteins such as lysozyme and lactoferrin inhibit bacterial growth. This evidence-based understanding underscores saliva’s multifaceted defense mechanisms against cavities. The intricate balance of saliva functions and enzyme activity is critical in oral health maintenance and cavity prevention.
Variations in Saliva Composition
Genetic factors greatly influence the composition of saliva, contributing to individual variability in cavity risk. Variations in saliva viscosity and mineral content are vital components affecting dental health. High viscosity may impede the flow of saliva, reducing its ability to clear food particles and neutralize acids, consequently increasing cavity susceptibility. Conversely, ideal viscosity promotes adequate oral clearance and protection against caries. Additionally, saliva’s mineral content, particularly calcium and phosphate ions, plays a pivotal role in enamel remineralization. Genetic predispositions impacting these mineral concentrations can either enhance or undermine enamel strength. Research indicates that genetic polymorphisms in salivary proteins and enzymes determine these factors, suggesting a significant heritable component. Understanding these genetic influences is essential for developing targeted preventive dental care strategies.
Genetic Variations and Their Influence on Oral Bacteria
Research indicates that genetic variations greatly influence the composition and behavior of oral bacterial communities, potentially affecting an individual’s susceptibility to cavities. Variations in genes associated with immune response and salivary proteins can alter the oral microbiome, creating environments either conducive or detrimental to pathogenic bacteria. Studies have shown that individuals with a genetic predisposition to certain bacterial strains, such as Streptococcus mutans, exhibit higher caries prevalence. These genetic factors modulate bacterial adhesion, biofilm formation, and acid production, contributing to enamel demineralization. Additionally, twin studies support the notion that genetic makeup profoundly influences the oral microbiome’s diversity and stability. Understanding these genetic determinants is vital for developing personalized dental care strategies aimed at optimizing oral health and preventing cavity formation.
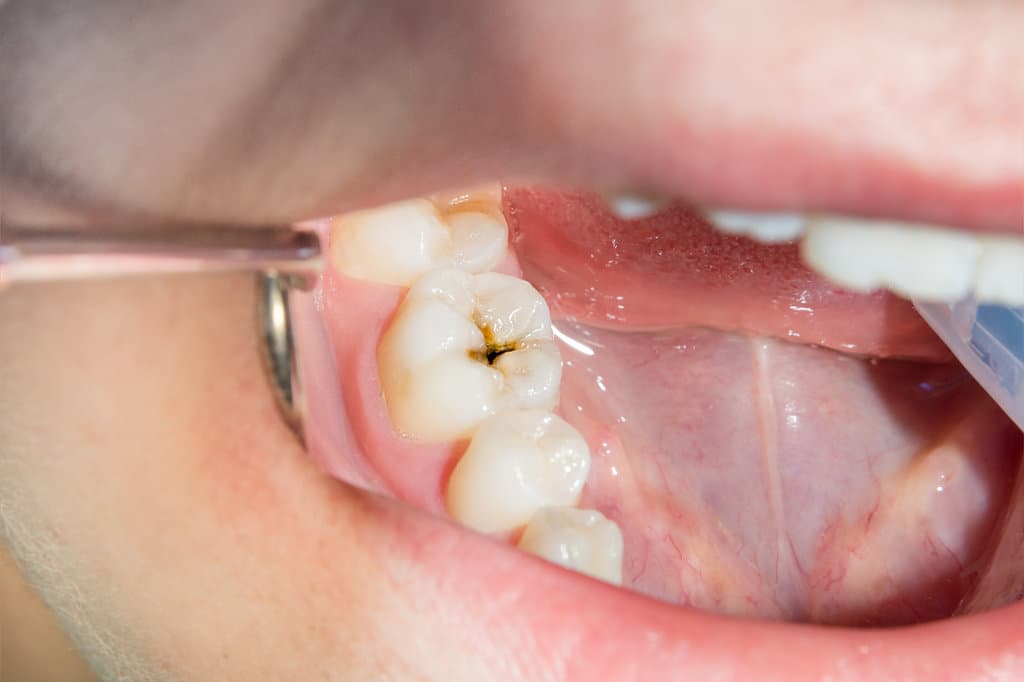
Hereditary Factors Affecting Tooth Shape and Alignment
While environmental factors play a role in dental development, hereditary factors primarily influence tooth shape and alignment. Tooth morphology, dictated by genetic determinants, affects the anatomical features such as cusp patterns, enamel thickness, and tooth size. These morphological traits are pivotal in determining susceptibility to dental caries. Alignment genetics further dictate the spatial arrangement of teeth within the dental arch, influencing occlusal relationships. Malocclusion, often genetically inherited, can predispose individuals to areas of plaque accumulation, increasing cavity risk. Studies employing genome-wide association approaches have identified genes linked to enamel formation and craniofacial development, illustrating the hereditary nature of these traits. Understanding these genetic influences aids in predicting dental health outcomes and tailoring preventive strategies for individuals based on their genetic predispositions.
The Interplay Between Diet, Lifestyle, and Genetics
Although genetic factors considerably influence dental health, the interaction between diet, lifestyle, and genetics creates a complex framework affecting cavity risk. Dietary habits, such as high sugar intake, can exacerbate genetic predispositions by facilitating bacterial growth responsible for dental caries. Lifestyle choices, including oral hygiene practices, additionally modulate this interaction. For instance, frequent brushing and flossing may mitigate the impact of genetic vulnerabilities. Evidence suggests that individuals with genetic variants associated with enamel hypoplasia are more susceptible to cavities, especially when compounded by poor dietary habits. Moreover, smoking and alcohol consumption have been identified as lifestyle factors that can exacerbate genetic susceptibility to cavities. Therefore, understanding the multifaceted relationship between these factors is essential for devising personalized dental care strategies.
Emerging Research on Genetic Markers for Dental Health
Advancements in genomic technologies have facilitated the identification of specific genetic markers that influence dental health, offering new insights into cavity risk assessment. Recent studies highlight a genetic predisposition to cavities linked to variations in genes associated with enamel formation, saliva composition, and immune response. Furthermore, genetic determinants are now recognized as influential in shaping the oral microbiome, which plays a critical role in cariogenic processes. Researchers have identified several polymorphisms that correlate with increased susceptibility to Streptococcus mutans colonization, a key bacterium in caries development. Evidence suggests that genetic interactions with environmental factors, such as diet and oral hygiene, can modulate the oral microbiome’s composition and function. This genetic understanding underscores the complexity of cavity etiology beyond traditional risk factors.
Implications for Personalized Dental Care and Prevention
The integration of genetic insights into dental care heralds a paradigm shift toward personalized preventive strategies. Genetic predisposition plays a critical role in individual susceptibility to dental caries, necessitating the development of tailored interventions. By employing genomic data, practitioners can identify patients at heightened risk and implement personalized prevention protocols that address specific vulnerabilities. Evidence suggests that genetic markers associated with enamel formation, salivary composition, and oral microbiota can inform targeted preventive measures. This approach enhances the efficacy of traditional methods, such as fluoride application and dietary modifications, by aligning them with genetic profiles. Ultimately, the fusion of genetic information with clinical practice offers a promising avenue for optimizing oral health outcomes and reducing the incidence of cavities through individualized care.
Frequently Asked Questions
Can Stress Levels Influence Genetic Predisposition to Cavities?
Stress response can modulate genetic expression related to oral health. Elevated stress levels may alter gene activity, potentially influencing susceptibility to cavities. Studies suggest that stress-induced hormonal changes could impact genes associated with enamel formation and saliva composition.
Are There Genetic Tests Available for Assessing Cavity Risk?
Genetic tests evaluating cavity risk focus on identifying specific genetic markers linked to tooth decay susceptibility. Analyzing these markers can guide personalized cavity prevention strategies, although the predictive accuracy and clinical utility of such tests require further validation.
How Does Genetics Affect the Effectiveness of Fluoride Treatments?
Genetic variants can influence fluoride absorption, potentially affecting the effectiveness of fluoride treatments. Studies suggest that specific genetic markers may alter enamel’s response to fluoride, indicating a personalized approach could optimize cavity prevention strategies and dental health outcomes.
Is There a Genetic Basis for Sensitivity to Dental Pain?
Research indicates a genetic basis for sensitivity to dental pain, identifying specific genetic markers associated with pain perception variability. These findings suggest that genetic predispositions can influence individual responses to dental stimuli, impacting overall pain management strategies.
Can Genetic Factors Influence the Success of Dental Implants?
Genetic inheritance can affect implant success by influencing bone density, immune response, and tissue regeneration. Studies suggest that individuals with specific genetic profiles may experience varied outcomes, indicating a potential role of genetics in dental implant efficacy.


